Ocrevus Relapse Rate
In 2017 Ocrelizumab became the first treatment to be approved for primary progressive MS PPMS a less common form in which the disease continually progresses without relapses. The average annualised relapse rate across all patients was very low 0005 and their mean change in Expanded Disability Status Scale score EDSS from baseline significantly improved from 171.
CADTH COMMON DRUG REVIEW Clinical Review Report for Ocrevus 6 Abbreviations ARR annualized relapse rate CDEC Canadian Drug Expert Committee CDP confirmed disability progression CDI confirmed disability improvement CI confidence interval CNS central nervous system DMT disease-modifying therapy EMA European Medicines Agency.
Ocrevus relapse rate. In the OPERA II trial the annualized relapse rate was 016 in the ocrelizumab group as compared with 029 in the interferon beta-1a group difference 014 annualized relapses. Treatment with Ocrevus significantly reduced the annualised relapse rate by 464 at 96 weeks compared with interferon beta-1a treatment in patients with RMS. You have fewer relapses than you might have had without treatment and disease progression is slowed.
The entire group on average experienced a very low annualized relapse rate of 0005. The average annualised relapse rate across all patients was very low 0005 and their mean change in Expanded Disability Status Scale score EDSS from baseline significantly improved from 171 to 155. Like Roches Ocrevus ocrelizumab ofatumumab targets a protein called CD20 thats present on the surface of immunological B cells.
About 10 percent of MS cases are diagnosed as. Most MS patients receive long-term DMTs that aim to slow the progression of disability decrease the relapse rate and maintain or improve the quality of life. Patients treated with Ocrevus in both the OPERA and extension trials also showed a lower annual relapse rate.
In clinical trials people taking Ocrevus had about 50 fewer relapses than people taking Rebif. No relapses worsening of disability or new or enlarging brain lesions with pre-specified MRI re-baselining at 8 weeks. Notably safety findings remained consistent across all OCREVUS exposure levels suggesting that higher exposure does not increase the.
Reduced relapse rates by nearly half vs Rebif 13132 OCREVUS demonstrated superior relapse reductions vs Rebif at Year 2 of the controlled period The efficacy and safety of OCREVUS in RMS were studied vs Rebif in 2 double-blind double-dummy trials evaluating over 1600 patients for 2 years. Patients given Ocrevus in both the OPERA studies and their extensions continued to show low annualized relapse rates that were sustained over the following two years 011 in year. OCREVUS reduced T1 gadolinium-enhancing and newenlarging T2 MRI lesions to nearly undetectable levels in RMS and PPMS patients and reduced annualized relapse rates to low levels 013-018 in RMS patients across all exposure segments.
Within the first eight weeks of treatment OCREVUS reduced the relapse rate by 55 percent compared with Rebif interferon beta-1a p00045 in a pooled exploratory analysis of the Phase III OPERA I and OPERA II studies in RMS. However the ones with the highest relevance to Covid-19 vaccines are those with B-cell-depleting mechanisms of action MOA such as GenentechRoches Ocrevus. Hauser served a similar role in an earlier phase 3 study of the first B-cell targeted MS treatment a Genentech drug called ocrelizumab brand name Ocrevus.
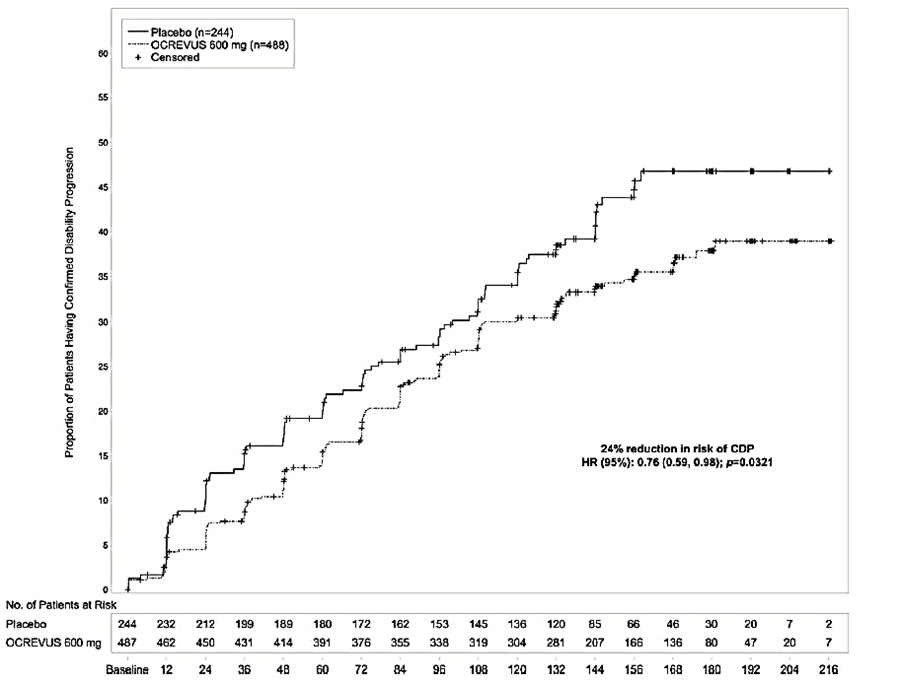
Ocrevus is a highly effective category 20 DMD. On the trials primary goal reducing the number of confirmed relapses ofatumumab handily surpassed Aubagio cutting annualized relapse rates by 51 and 59 respectively. For patients with PPMS treatment with Ocrevus led to a 24 reduction in the risk of 12-week confirmed disability progression compared with placebo.
Patients also showed a slight improvement on a disability. The average annualized relapse rate across all patients was very low 0005 and their mean change in Expanded Disability Status Scale score. After 48 weeks 85 of OCREVUS-treated patients achieved no evidence of disease activity NEDA.
There are now numerous DMTs approved in different forms of MS. The rate dropped from 0136 in the OPERA I. In a separate Phase II study in relapsing-remitting MS RRMS patients OCREVUS demonstrated rapid and near-complete suppression of brain MRI activity at eight.
 A Annualised Relapse Rate And B Confirmed Disability Progression In A Download Scientific Diagram
A Annualised Relapse Rate And B Confirmed Disability Progression In A Download Scientific Diagram
 Ms Progression Ocrevus Ocrelizumab
Ms Progression Ocrevus Ocrelizumab
 Ocrevus Ocrelizumab Efficacy For Relapsing Ms
Ocrevus Ocrelizumab Efficacy For Relapsing Ms
 The Ocrelizumab Phase Ii Extension Trial Suggests The Potential To Improve The Risk Benefit Balance In Multiple Sclerosis Multiple Sclerosis And Related Disorders
The Ocrelizumab Phase Ii Extension Trial Suggests The Potential To Improve The Risk Benefit Balance In Multiple Sclerosis Multiple Sclerosis And Related Disorders
 Ocrevus Ocrelizumab Efficacy For Relapsing Ms
Ocrevus Ocrelizumab Efficacy For Relapsing Ms
 Ocrelizumab Its Efficacy And Safety In Multiple Sclerosis Neurologia Com
Ocrelizumab Its Efficacy And Safety In Multiple Sclerosis Neurologia Com
 Onset Of Clinical And Mri Efficacy Of Ocrelizumab In Relapsing Multiple Sclerosis Neurology
Onset Of Clinical And Mri Efficacy Of Ocrelizumab In Relapsing Multiple Sclerosis Neurology
 Ocrevus Ocrelizumab Efficacy For Relapsing Ms
Ocrevus Ocrelizumab Efficacy For Relapsing Ms
 Ocrevus Ocrelizumab Efficacy For Relapsing Ms
Ocrevus Ocrelizumab Efficacy For Relapsing Ms
 Ocrevus Ocrelizumab Efficacy For Relapsing Ms
Ocrevus Ocrelizumab Efficacy For Relapsing Ms
 Ms Relapse Rates Go Even Lower In Ocrevus Extension Study
Ms Relapse Rates Go Even Lower In Ocrevus Extension Study
 Ocrevus Ocrelizumab Efficacy For Relapsing Ms
Ocrevus Ocrelizumab Efficacy For Relapsing Ms
 Ocrevus Ocrelizumab Results For Rms Relapsing Ms
Ocrevus Ocrelizumab Results For Rms Relapsing Ms
Comments
Post a Comment