Jnj Car T
1 LCAR-B38M identifies the investigational product. JNJ-68284528 JNJ-4528 is a chimeric antigen receptor T cell CAR-T therapy containing two BCMA-targeting single-domain antibodies designed to confer avidity.
 Car T Cell Therapy Johnson Johnson
Car T Cell Therapy Johnson Johnson
Bristol Myers Squibb and bluebird bio were first to file an anti-BCMA CAR-T for approval but Johnson Johnson and Legend Biotech are hot on their heels.

Jnj car t. In December 2017 JJ subsidiary Janssen Biotech Inc entered into the chimeric antigen receptor CAR T-cell space through a US350 million 304 million collaboration and licensing deal with Chinese firm Legend Biotech. JNJ-4528 is a structurally differentiated CAR-T with two BCMA-targeting single domain antibodies. Minimum 2 years after JNJ-68284528 infusion Day 1.
To gain access to a cancer cell therapy candidate developed by a previously little-known Chinese company called Legend Biotech. The first CAR-T therapy accepted by the Chinese regulator for review Legend has granted Janssen a worldwide licence to jointly develop and market JNJ-4528 in. Based in Nanjing China Legend Biotech wowed cancer researchers in June with an impressive late.
JNJ-68284528 is an autologous chimeric antigen receptor T-cell CAR-T therapy that targets B-cell maturation antigen BCMA a molecule expressed on the surface of mature B lymphocytes and malignant plasma cells. The BCMA-directed CAR T-cell therapy JNJ-4528 achieved a 100 overall response rate with early and deep responses in 29 patients with heavily pretreated relapsedrefractory myeloma. Immuno-oncology expert Iqbal Grewal PhD explains why the treatment has the potential to be a game changer for people with all types of cancer down the road.
Johnson Johnson has bought itself a foothold in the accelerating CAR-T field paying 350 million upfront through its subsidiary Janssen Biotech Inc. BCMA is highly expressed on myeloma cells and plasma cells. JNJ 16984 down 061.
The companies unveiled the. JJ has reported updated results from the CARTITUDE-1 study of its CAR-T candidate JNJ-4528 which was a hit at last years ASH congress after it. Patients T cells.
The deal vaults JJ into an intensely competitive. Number of Participants with Anti-JNJ-68284528 Antibodies Time Frame. A Study of JNJ-68284528 a Chimeric Antigen Receptor T Cell CAR-T Therapy Directed Against B-Cell Maturation Antigen BCMA in Participants With Relapsed or.
A phase IbII study of JNJ-4528 a B-cell maturation antigen BCMA-directed CAR. Johnson Johnson is renovating a facility in the US to make anti-cancer CAR-T candidate LCAR-B38M licensed from Legend Biotech last year. JNJ-4528 is a structurally differentiated CAR-T cell therapy containing a 4-1BB co- stimulatory domain and two BCMA-targeting single-domain antibodies designed to confer avidity.
13 Globally Legend Biotech together with Janssen. Multiple myeloma is a malignant plasma cell disorder diagnosed annually in approximately 86000 participants worldwide. Levels of JNJ-68284528 T cell expansion proliferation and persistence via monitoring CAR-T positive cell counts and CAR transgene level will be reported.
Its been in the news but what does CAR-T really mean for patients. These findings were announced in an oral presentation Update of CARTITUDE-1. 12 Proof of concept has been achieved in an investigator-initiated first-in-human study LEGEND-2 conducted in 74 patients with RRMM in China.
CAR-T therapies are a new type of cancer treatment and they have shown promising results so far. We present translational data from the phase 1b cohort of an ongoing phase 1b2 study conducted in the US to explore the safety and efficacy of JNJ-4528 in patients with R. JNJ-4528 LCAR-B38M is an investigational chimeric antigen receptor T cell CAR-T therapy for the treatment of patients with relapsed or refractory multiple myeloma.
The virtually unknown biotech wowed ASCO with early-stage data on its CAR-T and clearly caught JJs attention. JNJ-4528 is a chimeric antigen receptor T cell CAR-T therapy containing two B-cell maturation antigen BCMA-targeting single-domain antibodies designed to confer avidity. LCAR-B38MJNJ-4528 Our first product candidate LCAR-B38MJNJ-4528 is a B cell maturation antigen BCMA-directed CAR-T cell therapy.
Janssens investigational CAR T-cell therapy for multiple myeloma called JNJ-4528 continues to lead to strong and durable responses in all patients with relapsed or refractory disease who are participating in a Phase 1b2 trial. 2 Longer-term follow-up results from the Phase 1b portion of the study n29 to be shared in an oral presentation at. The site youre being redirected to is a branded pharmaceutical website.
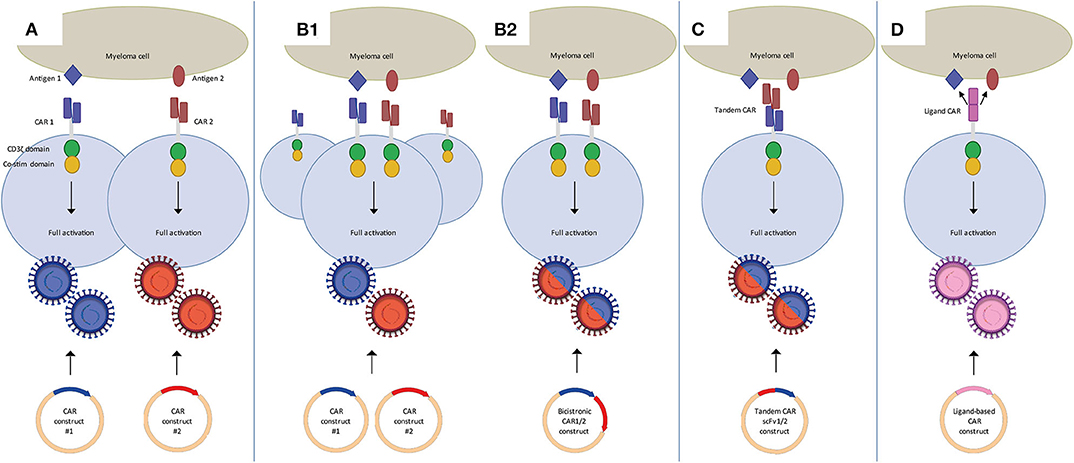 Frontiers Dual Targeting To Overcome Current Challenges In Multiple Myeloma Car T Cell Treatment Oncology
Frontiers Dual Targeting To Overcome Current Challenges In Multiple Myeloma Car T Cell Treatment Oncology
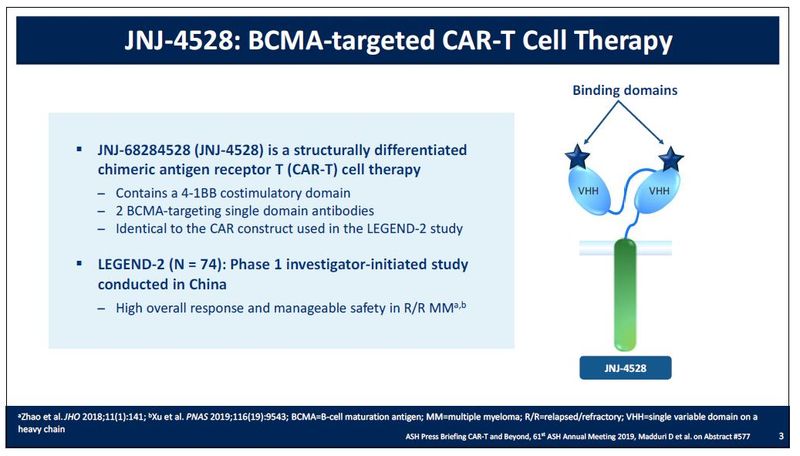 Janssen Presents Initial Results For Bcma Car T Therapy Jnj 4528 Showing Early Deep And High Responses In The Treatment Of Relapsed Or Refractory Multiple Myeloma Cliniexpert
Janssen Presents Initial Results For Bcma Car T Therapy Jnj 4528 Showing Early Deep And High Responses In The Treatment Of Relapsed Or Refractory Multiple Myeloma Cliniexpert
 The Next Frontier In Cancer Care What Is Car T Cell Therapy Johnson Johnson
The Next Frontier In Cancer Care What Is Car T Cell Therapy Johnson Johnson
 Car T Cell Therapy Johnson Johnson
Car T Cell Therapy Johnson Johnson
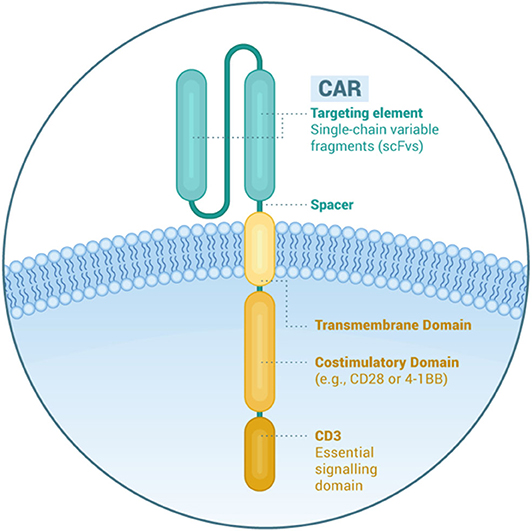 Frontiers Car T Cells In Multiple Myeloma State Of The Art And Future Directions Oncology
Frontiers Car T Cells In Multiple Myeloma State Of The Art And Future Directions Oncology
 Janssen S Bcma Car T Therapy Jnj 4528 Showed Early Deep And Durable Responses In Heavily Pretreated Patients With Multiple Myeloma Business Wire
Janssen S Bcma Car T Therapy Jnj 4528 Showed Early Deep And Durable Responses In Heavily Pretreated Patients With Multiple Myeloma Business Wire
 Ash 2019 Results From The Cartitude 1 Trial Int Myeloma Fn
Ash 2019 Results From The Cartitude 1 Trial Int Myeloma Fn
 Igniting The Future Of Cell Therapy
Igniting The Future Of Cell Therapy
 Bertrand Delsuc On Twitter Kalos Janssen Jnj A New Kid On The Blocks After The Bcma Car T From Legend Bio China Tgting 2 Bcma Epitopes Cartmeeting Contrary To What Is Reported Usually
Bertrand Delsuc On Twitter Kalos Janssen Jnj A New Kid On The Blocks After The Bcma Car T From Legend Bio China Tgting 2 Bcma Epitopes Cartmeeting Contrary To What Is Reported Usually
 Car T Cell Therapy Johnson Johnson
Car T Cell Therapy Johnson Johnson
 Igniting The Future Of Cell Therapy
Igniting The Future Of Cell Therapy
 Uzivatel Bertrand Delsuc Na Twitteru D Madduri Phase 1b Results From Cartitude 1 A Phase 1b 2 Study Of Jnj 4528 A Chimeric Antigen Receptor T Car T Cell Therapy Directed Against B Cell Maturation Antigen
Uzivatel Bertrand Delsuc Na Twitteru D Madduri Phase 1b Results From Cartitude 1 A Phase 1b 2 Study Of Jnj 4528 A Chimeric Antigen Receptor T Car T Cell Therapy Directed Against B Cell Maturation Antigen
 Phase Ib Ii Cartitude 1 Capsule Summary Slidesets Myeloma 2019 Ash Annual Meeting Oncology Clinical Care Options
Phase Ib Ii Cartitude 1 Capsule Summary Slidesets Myeloma 2019 Ash Annual Meeting Oncology Clinical Care Options
 Ash18 J J Legend Car T Treatment Posts 88 Orr In Multiple Myeloma Fiercebiotech
Ash18 J J Legend Car T Treatment Posts 88 Orr In Multiple Myeloma Fiercebiotech
Comments
Post a Comment