Epclusa Treatment Duration
Your cost might vary based on insurance or manufacturer-provided discounts. Hepatitis C virus occurs in several varieties genotypes and Epclusa can be used to treat hepatitis C caused by all genotypes of the virus.
 Direct Acting Antivirals And Ribavirin For Hepatitis C Virus Treatment Download Table
Direct Acting Antivirals And Ribavirin For Hepatitis C Virus Treatment Download Table
If future studies continue to demonstrate effective HCV cure with shorter 6-week treatment duration.
Epclusa treatment duration. A quantity of 84 tablets per 365 days will be covered without prior authorization. Epclusa is available as tablets containing sofosbuvir and velpatasvir 400 mg 100 mg and 200 mg 50 mg. This is enough drug for individuals to complete a 12 week course of therapy.
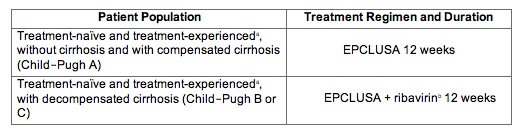
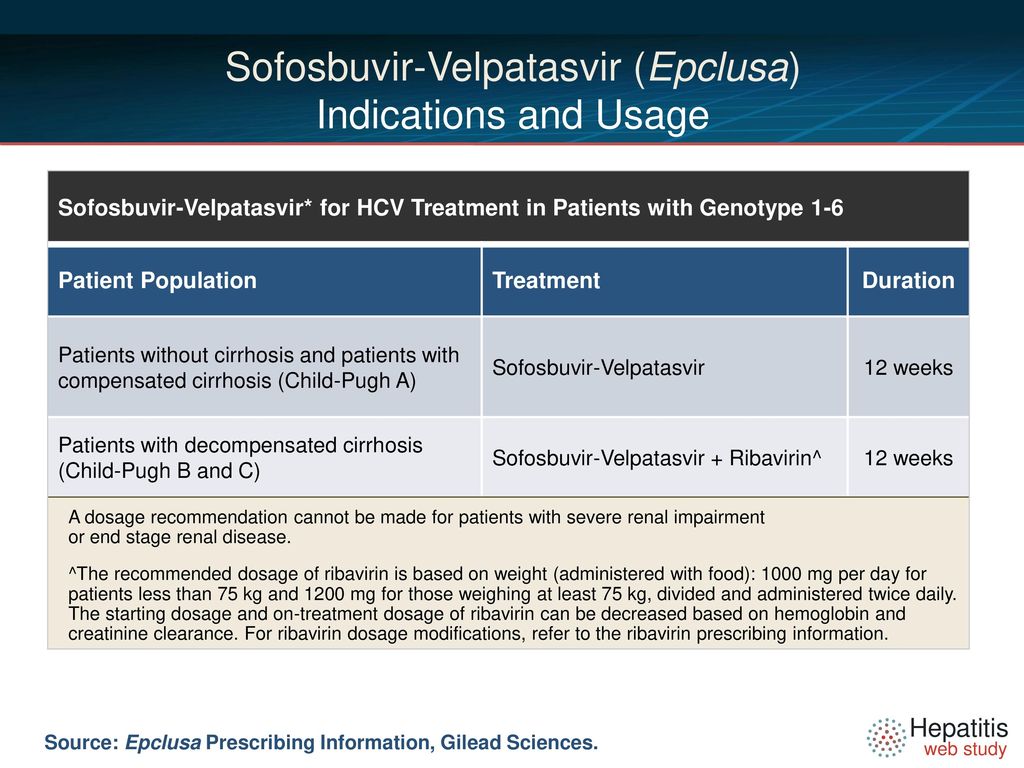
For treatment-naïve and treatment-experienced liver transplant recipients without cirrhosis or with compensated cirrhosis Child-Pugh A the recommended regimen is EPCLUSA once daily for 12 weeks see Clinical Studies 143 and 145. The primary efficacy endpoint was the proportion of patients achieving sustained virologic response at 12 weeks after the cessation of treatment. In patients with decompensated cirrhosis Child-Pugh B or C Epclusa should be administered with weigh-based ribavirin.
____ weeks Test for Cure Date. Do not consider pregnancy until at least 6 months after treatment of either partner has ended Store at room temperature 86F Daily Medication Schedule. Randomisation was stratified by the presence or absence of cirrhosis and prior treatment.
Epclusa for 12 weeks resulted in an overall SVR12 of 83 which increased to 94 in those assigned Epclusa RBV for 12 weeks Curry et al 2015. _____ Expected Treatment Duration. ASTRAL-3 was a randomised open-label study that evaluated 12 weeks of treatment with Epclusa compared with 24 weeks of treatment with SOFRBV in patients with genotype 3 HCV infection.
For an indication or condition addressed as an approval in the above criteria section approve the duration described above to complete a course therapy eg a individual who should receive 12 weeks and has received 3. Epclusa x 12 weeks Decompensated Child-Pugh B or C Cirrhosis Epclusa ribavirin x 12 weeks. A fifth trial ASTRAL-5 in 106 HIVHCV co-infected patients showed that open-label treatment with Epclusa for 12 weeks resulted in an SVR12 of 95 99104 Bräu et al 2016.
O Females who take EPCLUSA in combination with ribavirin should avoid becoming pregnant during treatment and for 6 months after stopping treatment. With the exception of asthenia each of these adverse reactions. Epclusa is administered as one tablet taken orally once daily with or without food.
The recommended treatment duration is 12 weeks in all individuals. Patients were randomised in a 11 ratio to treatment with Epclusa for 12 weeks or SOFRBV for 24 weeks. Of subjects receiving EPCLUSA who experienced these adverse reactions 79 had an adverse reaction of mild severity Grade 1.
The recommended dose of Epclusa is one tablet taken orally once daily with or without food see section 52. The recommended dose in adults is one 400100 mg tablet taken once a day for 12 weeks. Epclusa is covered by most insurance plans.
Immediate treatment of acute HCV with DAAs can improve clinical outcomes and be highly cost-effective or cost-saving compared with deferring treatment until the chronic phase of infection. Individual Has Been Started on Epclusa brand or generic. Equal to 10 all grades observed with treatment with EPCLUSA for 12 weeks are headache and fatigue.
For coverage of additional quantities for example a. ____ pm Epclusa Take 1 tablet daily. Call your healthcare provider right away if you think you may be pregnant or become pregnant during treatment with EPCLUSA in combination with ribavirin.
Receiving 12 weeks of treatment with EPCLUSA in ASTRAL-1 include headache 22 fatigue 15 nausea 9 asthenia 5 and insomnia 5. Borgia and colleagues enrolled 59 patients with HCV and ESRD to undergo 12 weeks of treatment with Epclusa sofosbuvirvelpatasvir Gilead Sciences. The cost of Epclusa sofosbuvir and velpatasvir is roughly US78078 for a full 12-week course of treatment.
The ASTRAL-studies treatment with Epclusa RBV for 24 weeks can be considered for patients who have failed therapy on an NS5A-containing regimen and who are deemed at high risk for clinical disease progression and who do not have alternative treatment options. 61 The most common adverse reactions incidence greater than or equal to 10 all grades. Recommended treatment and duration for all HCV genotypes Patient populationa Treatment and duration Patients without cirrhosis and patients.
In patients at risk of transmitting HCV treating acute HCV became cost-saving increasing QALYs by 003 and decreasing costs by 3655.
 European Commission Grants Marketing Authorization For Gilead S Epclusa Sofosbuvir Velpatasvir For The Treatment Of All Genotypes Of Chronic Hepatitis C Gilead
European Commission Grants Marketing Authorization For Gilead S Epclusa Sofosbuvir Velpatasvir For The Treatment Of All Genotypes Of Chronic Hepatitis C Gilead
 Core Concepts Treatment Of Hcv Genotype 3 Treatment Of Chronic Hepatitis C Infection Hepatitis C Online
Core Concepts Treatment Of Hcv Genotype 3 Treatment Of Chronic Hepatitis C Infection Hepatitis C Online
![]() Chronic Hepatitis C Chc Treatments By Genotype Pharmacy Investigators Consultants
Chronic Hepatitis C Chc Treatments By Genotype Pharmacy Investigators Consultants
 Revisions To The Epclusa Label Approved Coinfection Approval
Revisions To The Epclusa Label Approved Coinfection Approval
 Challenges And Perspectives Of Direct Antivirals For The Treatment Of Hepatitis C Virus Infection Journal Of Hepatology
Challenges And Perspectives Of Direct Antivirals For The Treatment Of Hepatitis C Virus Infection Journal Of Hepatology
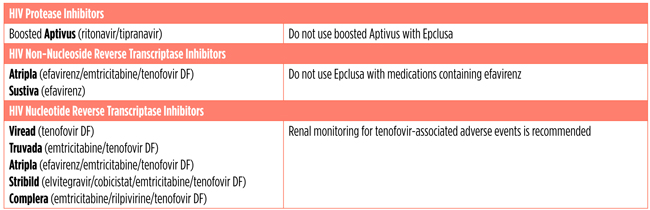 Epclusa Fact Sheet Treatment Action Group
Epclusa Fact Sheet Treatment Action Group
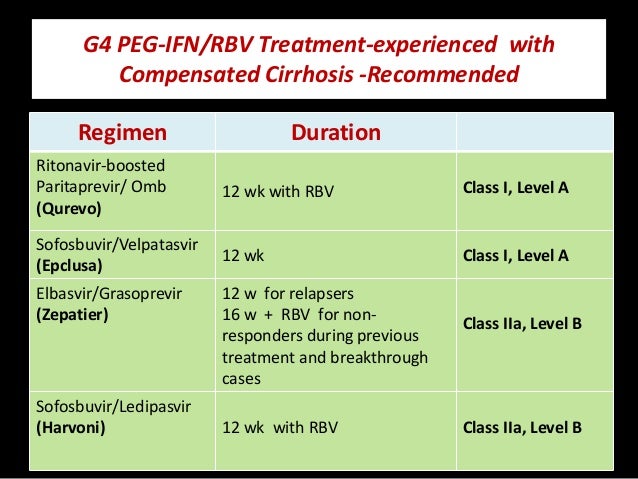 Revision Of Treatment Protocols For Hcv Genotype 4 Infection 2016
Revision Of Treatment Protocols For Hcv Genotype 4 Infection 2016
 Sofosbuvir Velpatasvir Epclusa Ppt Download
Sofosbuvir Velpatasvir Epclusa Ppt Download
 Epclusa Fact Sheet Treatment Action Group
Epclusa Fact Sheet Treatment Action Group
Http Www Who Int Selection Medicines Committees Expert 21 Applications S6 Sofosbuvir Velpatasvir Add 2 Pdf
 Epclusa Approved For Chronic Hepatitis C Mymatrixx
Epclusa Approved For Chronic Hepatitis C Mymatrixx
Https Cdn Ymaws Com Www Az Osteo Org Resource Resmgr Convention Handouts 2019 Presentation Abbott Brand Pdf
 Direct Acting Antivirals And Ribavirin For Hepatitis C Virus Treatment Download Table
Direct Acting Antivirals And Ribavirin For Hepatitis C Virus Treatment Download Table
Comments
Post a Comment