Hemophilia Clinical Trials
Have hemophilia B with 2 IUdL 2 endogenous FIX activity levels as documented by a certified clinical laboratory at the time of screening. We obtained HJHS scores from 107 PwHA.
 Clinical Trials For Gene Therapy Of Hemophilia Download Table
Clinical Trials For Gene Therapy Of Hemophilia Download Table
A surge in therapeutic clinical trials over recent years is paving the way for transformative treatment options for patients with hemophilia.

Hemophilia clinical trials. Individuals with hemophilia B suffer repeated bleeding events which can cause chronic joint disease and sometimes leads to death due to the inability for blood to clot efficiently. We are committed to advancing hemophilia patient care and facilitating the development of novel treatments through research studies and clinical trials. The goal of this research is to study the use of hemophilia treatment.
The Inhibitor Prevention Trial is a 48-week randomized phase III trial in which 66 previously untreated patients PUPs with severe hemophilia. A Trial Comparing Nonacog Beta Pegol N9-GP and ALPROLIX in Patients With Haemophilia B Rochester MN This trial is conducted in Europe and the United States of America. The study drug is identified as AAV5-hFIXco-Padua AMT- 061.
This study will capture different approaches in the management of persons with haemophilia A HA and inhibitors. 36 An initial patient treated at a low vector dose had nearly undetectable FVIII levels with little change in. The aim of this trial is to compare the pharmacokinetics the exposure of the trial drug in the body of nonacog beta pegol N9-GP and ALPROLIX in patients with haemophilia B.
A randomized clinical trial of prophylaxis in children with hemophilia A the ESPRIT Study This randomized trial confirms the efficacy of prophylaxis in preventing bleeds and arthropathy in children with hemophilia particularly when it is initiated early in life. HA is a serious blood coagulation disorder caused by a deficiency in FVIII that results in a failure to produce FVIII in sufficient quantities to achieve satisfactory haemostasis. In this study we explored the potential effect of emicizumab prophylaxis on joint health scores and biomarkers of bone health in PwHA without factor VIII FVIII inhibitors who participated in the HAVEN 3 clinical trial.
Therapies engineered to prolong clotting factor protein circulation time manipulate the balance of pro-coagulant and anti-coagulant proteins or introduce new genetic material to enable endogenous factor protein production dominate the clinical trial landscape of hemophilia. The purpose of this study is to determine the frequency of bleeding episodes in patients receiving fitusiran as prophylactic treatment of hemophilia compared with patients who are assigned to continue with their regular medication. The INHIBIT Clinical Trials Platform includes two linked trials the Inhibitor Prevention Trial Prevention Trial and the Inhibitor Eradication Trial Eradication Trial that will be conducted at up to 41 US.
The hemophilia treatment center HTC physician and participant will decide on the FDA-approved treatment to be used which may include non-factor products bypassing agents or clotting factor replacement products. The current single country multi-centric open label non-randomized pragmatic clinical trial is a post-approval study to fulfill the Central Drugs Standard Control Organization CDSCO request for supplementary information relating to the use of moroctocog. In addition the study will assess safety quality of life pharmacodynamics PD and pharmacokinetics PK.
The introduction of recombinant factor concentrates in the early 1990s facilitated the use of prophylactic replacement as standard care for hemophilia rather than on-demand treatment. This chronic joint disease can have significant physical psychosocial and quality-of-life effects including financial burden. Hemophilia treatment centers HTCs affiliated with universities.
Milwaukee June 16 2020 Patients are now able to participate in a phase I first-in-human FDA approved clinical trial seeking a potential long-term treatment for Severe Hemophilia A using a gene therapy that targets synthesis of coagulation Factor Eight FVIII which is stored and released from blood platelets at the site of an injured blood vessel. The development of new strategies including disease-modifying and curative therapies form the basis of our progressive approach to hemophilia management for patients at Weill Cornell Medicine. If the screening result is 2 due to insufficient washout from FIX protein product then the severity of hemophilia B may be confirmed by documented historical evidence from a certified clinical laboratory demonstrating 2 FIX coagulant activity.
C3731003 is a pivotal Phase 3 study to evaluate the clinical efficacy and safety of a single IV infusion of PF-07055480 Recombinant AAV26 Human Factor VIII Gene Therapy in adult male participants with moderately severe or severe hemophilia A FVIIIC1 for the study duration of 5 years. Trial of AMT-061 in Severe or Moderately Severe Hemophilia B Patients in progress not accepting new patients This is an open-label single-dose multi-center multinational trial to demonstrate the efficacy of AMT-061 and to further describe its safety profile. Hemophilia B or Christmas disease is a genetic bleeding disorder resulting in the lack of ability to produce blood-clotting factor IX FIX.
Hemophilia A clinical trials BioMarin initiated the first clinical trial for hemophilia A using liver gene transfer of an AAV5 vector expressing a codon optimized BDD-FVIII-SQ protein BMN 270. The study will follow people with hemophilia A or B from across the country for about 4 years as they receive treatment. Measurement properties of the Haem-A-QoL in haemophilia clinical trials These psychometric analyses provide evidence of the reliability validity and ability to detect change of the Haem-A-QoL to assess the HRQoL of adult males with severe haemophilia A and B in longitudinal clinical trials.
The clinical hold was initiated following the submission of a safety report in mid-December relating to a possibly related serious adverse event associated with a preliminary diagnosis of hepatocellular carcinoma HCC a form of liver cancer in one patient in the HOPE-B trial that was treated with etranacogene dezaparvovec AMT-061 in October 2019. The Hemophilia Joint Health Score HJHS is a tool used to assess joint function and gait with a lower score indicating better joint health.
 Fda Guidance For Human Gene Therapy For Hemophilia A B
Fda Guidance For Human Gene Therapy For Hemophilia A B
 Kovaltry Recombinant Factor Viii Octocog Alfa For The Treatment Of Hemophilia A Pharmacokinetics
Kovaltry Recombinant Factor Viii Octocog Alfa For The Treatment Of Hemophilia A Pharmacokinetics
 Clinical Trials For Gene Therapy Of Hemophilia Download Table
Clinical Trials For Gene Therapy Of Hemophilia Download Table
 Recently Approved Or In Clinical Trial Novel Hemophilia A Therapeutics Download Table
Recently Approved Or In Clinical Trial Novel Hemophilia A Therapeutics Download Table
 Hemophilia Clinical Trials Free Whitepaper
Hemophilia Clinical Trials Free Whitepaper
Hemophilia Therapy The Future Has Begun Haematologica
 New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
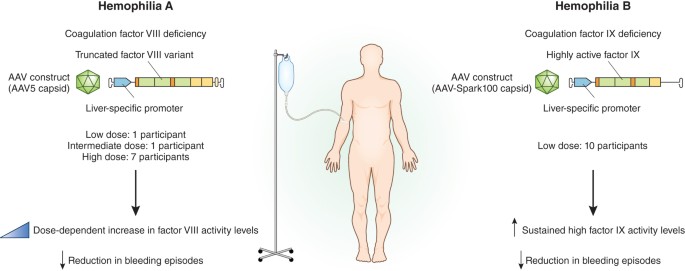 Gene Therapies For Hemophilia Hit The Mark In Clinical Trials Nature Medicine
Gene Therapies For Hemophilia Hit The Mark In Clinical Trials Nature Medicine
 Randomized Clinical Trials Concerning Rfviia In Hemophilia Patients Download Table
Randomized Clinical Trials Concerning Rfviia In Hemophilia Patients Download Table
 A Molecular Revolution In The Treatment Of Hemophilia Sciencedirect
A Molecular Revolution In The Treatment Of Hemophilia Sciencedirect
 New Gene Therapy Cme Activity Updates On Clinical Trials Of Gene Therapy In Hemophilia Hemophilia World News
New Gene Therapy Cme Activity Updates On Clinical Trials Of Gene Therapy In Hemophilia Hemophilia World News
 Preclinical Studies And Clinical Trials On Gene And Cell Therapy For Download Table
Preclinical Studies And Clinical Trials On Gene And Cell Therapy For Download Table
Gene Editing Drugs Clinical Potential And Important Regulatory Considerations Biopharma Excellence
 New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
New Gene Therapy For Hemophilia Elearning Module Hemophilia World News
Comments
Post a Comment